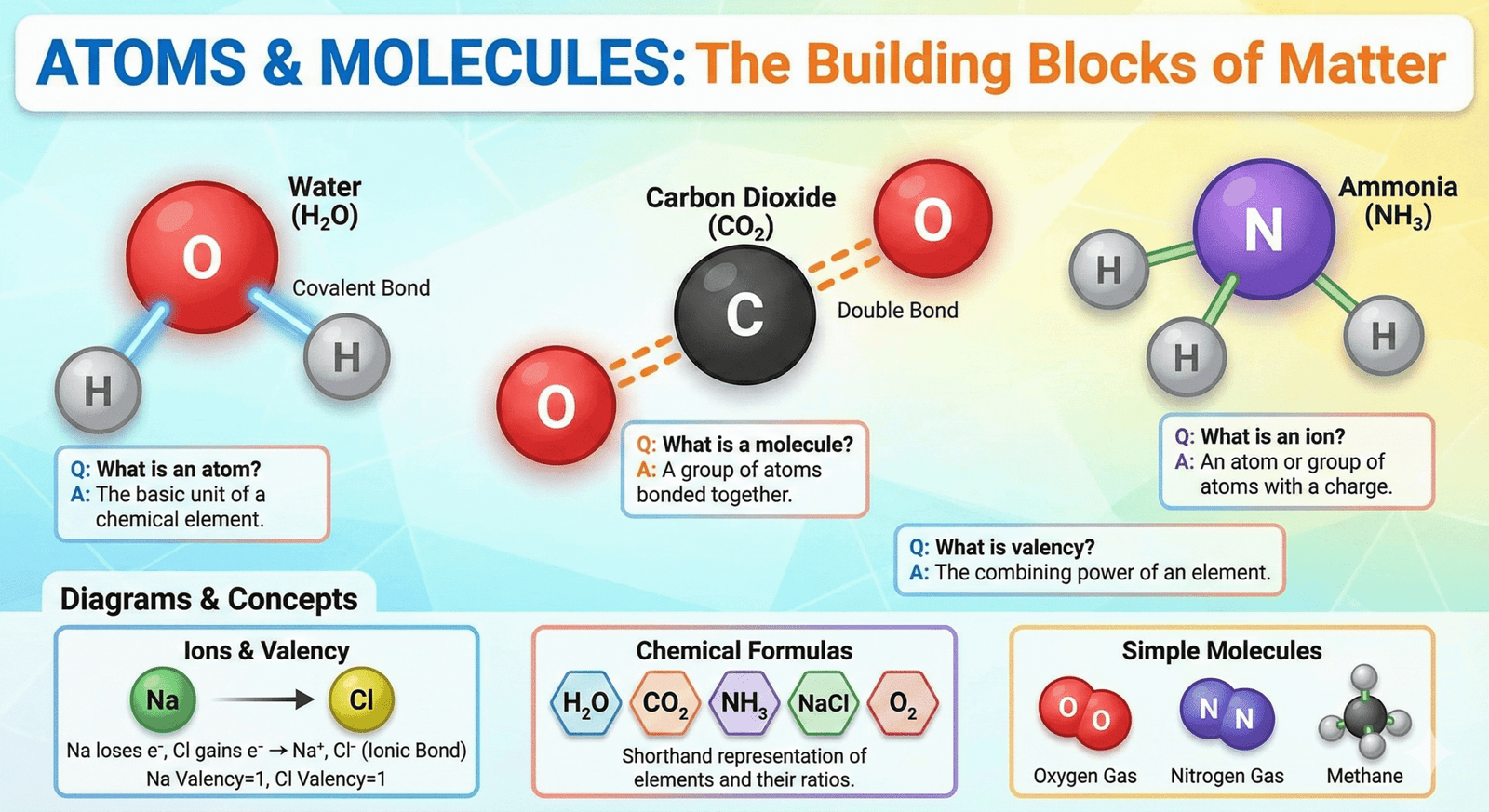
If you are preparing for Class 9 Science exams, this list of important questions and answers from Chapter 3: Atoms and Molecules will help you score full marks.
Below are short answer questions, numericals, valency-based questions, long answer questions, and comparison tables.
Table of Contents
Most Important Questions & Answers
1. Laws of Chemical Combination
1. State the Law of Conservation of Mass with an example.
Answer: The Law of Conservation of Mass states that in a chemical reaction, the total mass of the reactants is always equal to the total mass of the products. This means mass cannot be created or destroyed during a chemical reaction; it only changes from one form to another.
Example: When sodium carbonate (Na₂CO₃) reacts with acetic acid (CH₃COOH), carbon dioxide, water, and sodium acetate are formed. If we weigh the reactants and products carefully, the total mass remains the same.
2. State the Law of Constant (Definite) Proportions.
Answer: The Law of Definite Proportions states that a chemical compound always contains the same elements in the same fixed ratio by mass, no matter the source or amount of the compound.
Example: Water always consists of hydrogen and oxygen in a mass ratio of 1:8. Whether it is from a river or formed in a laboratory, this ratio does not change.
3. Which postulate of Dalton’s atomic theory explains the Law of Conservation of Mass?
Answer: The postulate “Atoms are indivisible and cannot be created or destroyed” explains this law. Since atoms are the building blocks of matter, their total number remains constant during chemical reactions, so mass remains unchanged.
4. Which postulate of Dalton’s atomic theory explains the Law of Constant Proportions?
Answer: The postulate “Atoms of different elements combine in fixed ratios to form compounds” explains this law. Compounds are formed by combining atoms in definite whole-number ratios, which ensures that the proportion by mass of elements in a compound is always fixed.
2. Dalton’s Atomic Theory
4. Explain Dalton’s Atomic Theory.
Answer: Dalton’s Atomic Theory states:
- All matter is made up of very tiny indivisible particles called atoms.
- Atoms of a particular element are identical in mass and properties.
- Atoms of different elements have different masses and properties.
- Atoms combine in simple whole-number ratios to form compounds.
- Chemical reactions involve the rearrangement of atoms; atoms are neither created nor destroyed.
This theory explains the laws of chemical combination, including the Law of Conservation of Mass and the Law of Definite Proportions.
3. Atom, Molecule & Atomicity
5. Define atomic mass unit (u).
Answer: An atomic mass unit (u) is defined as one-twelfth (1/12) of the mass of a single atom of carbon-12. It is a standard unit used to express the mass of atoms and molecules, because atoms are extremely small and their mass cannot be measured in grams conveniently.
6. Why can’t we see atoms with naked eyes?
Answer: Atoms are extremely tiny, with a radius of about 10⁻¹⁰ meters, which is much smaller than the wavelength of visible light. Our eyes cannot detect objects this small, so special instruments like electron microscopes are required to "see" atoms indirectly.
7. What is a molecule? Give examples.
Answer: A molecule is the smallest particle of an element or compound that can exist independently while retaining all its chemical properties. Molecules are made of one or more atoms chemically bonded together.
Examples: Oxygen (O₂) is a molecule made of two oxygen atoms; water (H₂O) is a molecule made of two hydrogen atoms and one oxygen atom.
8. What is atomicity? Give examples.
Answer: Atomicity refers to the number of atoms present in a molecule.
| Substance | Atomicity | Explanation |
|---|---|---|
| Helium (He) | 1 | Monoatomic, as it exists as single atoms naturally |
| Oxygen (O₂) | 2 | Diatomic, as each molecule has 2 atoms of oxygen |
| Ozone (O₃) | 3 | Triatomic, as each molecule has 3 oxygen atoms |
4. Numerical Questions
9. Calculate molecular mass of CO₂.
Answer: The molecular mass of a compound is the sum of the atomic masses of all atoms in its molecule.
C = 12, O = 16
Molecular mass = 12 + 2×16 = 44 u
10. Calculate formula unit mass of CaCl₂.
Answer: Formula unit mass is the sum of the atomic masses of all atoms in a formula unit.
Ca = 40, Cl = 35.5
Formula unit mass = 40 + 2×35.5 = 40 + 71 = 111 u
11. Hydrogen and oxygen combine in 1:8 ratio by mass. What mass of oxygen reacts with 3 g hydrogen?
Answer: 1 g of hydrogen reacts with 8 g of oxygen. Therefore, 3 g of hydrogen will react with 3×8 = 24 g of oxygen.
12. A compound contains 0.096 g boron and 0.144 g oxygen. Calculate % composition.
Answer:
Total mass = 0.096 + 0.144 = 0.24 g
%B = (0.096 / 0.24) × 100 = 40%
%O = (0.144 / 0.24) × 100 = 60%
5. Valency & Chemical Formulas
13. Write the chemical formula for magnesium chloride.
Answer: Magnesium has a valency of 2 and chlorine has a valency of 1. By crossing over the valencies, the formula becomes MgCl₂.
14. Write the formula of aluminium oxide.
Answer: Aluminium has a valency of 3, oxygen has a valency of 2. Crossing over gives Al₂O₃.
15. Write the formula for ammonium sulphate.
Answer: Ammonium (NH₄⁺) has valency 1, sulphate (SO₄²⁻) has valency 2. Crossing over gives (NH₄)₂SO₄.
6. Short Important Questions
16. What are polyatomic ions? Give examples.
Answer: Polyatomic ions are ions that consist of a group of atoms bonded together that collectively carry a net positive or negative charge.
Examples: Sulphate (SO₄²⁻), Nitrate (NO₃⁻)
17. Difference between atom and molecule:
| Atom | Molecule |
|---|---|
| Smallest particle of an element | Smallest particle of an element or compound |
| Cannot exist independently | Can exist independently |
| Made of only 1 atom | Made of 2 or more atoms chemically bonded |
| Example: He, Na | Example: O₂, H₂O, CO₂ |
18. What is meant by a chemical formula?
Answer: A chemical formula is a symbolic representation that shows which elements are present in a compound and the number of atoms of each element. For example, H₂O shows two hydrogen atoms and one oxygen atom.
19. What are ions? Give examples.
Answer: Ions are charged particles formed when an atom or group of atoms loses or gains electrons.
Examples: Sodium ion (Na⁺), Chloride ion (Cl⁻)
20. Why are brackets used in formulas such as Ca(OH)₂?
Answer: Brackets are used to indicate that a group of atoms is repeated in the formula. In Ca(OH)₂, there are two hydroxide (OH⁻) groups combined with one calcium (Ca²⁺) atom.
7. Comparison Tables
Atom vs Molecule
| Atom | Molecule |
|---|---|
| Smallest particle of an element | Smallest particle of an element or compound |
| Cannot exist independently | Can exist independently |
| Made of 1 atom | Made of 2+ atoms |
| Example: He, Na | Example: O₂, H₂O, CO₂ |
Element vs Compound
| Element | Compound |
|---|---|
| Made of only one kind of atom | Made of two or more elements chemically combined |
| Cannot be broken down chemically | Can be broken down chemically |
| Properties same throughout | Properties different from constituent elements |
| Example: O₂, Fe | Example: H₂O, CO₂, NaCl |
Some Important Tables Revision
Table 3.1: Symbols for some elements
| Element | Symbol | Element | Symbol | Element | Symbol |
|---|---|---|---|---|---|
| Aluminium | Al | Copper | Cu | Nitrogen | N |
| Argon | Ar | Fluorine | F | Oxygen | O |
| Barium | Ba | Gold | Au | Potassium | K |
| Boron | B | Hydrogen | H | Silicon | Si |
| Bromine | Br | Iodine | I | Silver | Ag |
| Calcium | Ca | Iron | Fe | Sodium | Na |
| Carbon | C | Lead | Pb | Sulphur | S |
| Chlorine | Cl | Magnesium | Mg | Uranium | U |
| Cobalt | Co | Neon | Ne | Zinc | Zn |
Table 3.2: Atomic masses of a few elements
| Element | Atomic Mass (u) |
|---|---|
| Hydrogen | 1 |
| Carbon | 12 |
| Nitrogen | 14 |
| Oxygen | 16 |
| Sodium | 23 |
| Magnesium | 24 |
| Sulphur | 32 |
| Chlorine | 35.5 |
| Calcium | 40 |
Names and symbols of some ions
| Valency | Name of Ion | Symbol | Non-Metallic Ions | Symbol | Polyatomic Ion | Symbol |
|---|---|---|---|---|---|---|
| 1 | Sodium | Na+ | Hydrogen | H+ | Ammonium | NH4+ |
| Potassium | K+ | Hydride | H- | Hydroxide | OH– | |
| Silver | Ag+ | Chloride | Cl- | Nitrate | NO3– | |
| Copper (I)* | Cu+ | Bromide | Br- | Hydrogen carbonate | HCO3– | |
| Iodide | I– | Carbonate | CO3 2– | |||
| 2 | Magnesium | Mg2+ | Oxide | O2– | Sulphite | SO3 2– |
| Calcium | Ca2+ | Sulphide | S2– | Sulphate | SO4 2– | |
| Zinc | Zn2+ | |||||
| Iron (II)* | Fe2+ | |||||
| Copper (II)* | Cu2+ | |||||
| 3 | Aluminium | Al3+ | Nitride | N3– | Phosphate | PO4 3– |
| Iron (III)* | Fe3+ |
Relevant Posts:
- CBSE Class 9 Sound Chapter Important Questions for Exams (NCERT Focused)
- CBSE Class 9 Science Chapter 10 Work and Energy Important Questions & Answers
- Improvement in Food Resources: CBSE Class 9 Science Important Questions and Answers
- Structure of the Atom – Important Questions and Answers for CBSE Class 9
- Atoms and Molecules Class 9 – Most Important Questions and Answers
- CBSE Class 9 Computer Applications Syllabus 2025-26: Download PDF & Full Exam Pattern
- CBSE Class 9 Hindi B Syllabus 2025-26: Download PDF & Full Exam Pattern
- CBSE Class 9 Hindi A Syllabus 2025-26: Download PDF & Full Exam Pattern
- CBSE Class 9 English Syllabus 2025-26: Download PDF & Full Exam Pattern
- CBSE Class 9 SST Syllabus 2025-26: Download PDF & Full Exam Pattern
- CBSE Class 9 Science Syllabus 2025-26: Download PDF & Full Exam Pattern
- CBSE Class 9 Maths Syllabus 2025-26: Download PDF & Full Exam Pattern
- Gravitation Class 9 Important Questions
- Force and Laws of Motion Class 9 Important Questions