Important questions of Matter in Our Surroundings Class 9 Science Chapter 1
In this article, you will get all the important questions from the chapter Matter in Our Surroundings Class 9. These questions compiled by CBSE Guidance must be gone through before appearing in the exam.
Q. No. 1) Convert -50°C into Kelvin scale.
Ans. -50 + 273 = 223K
Q. No. 2) When we put salt in water, it dissolves. Where do the salt particles disappear?
Ans. When we dissolve salt in water, the particles of salt get into the spaces between particles of water.
Q. No. 3) When we throw water on the floor first it starts spreading but after some time it stops. Give reasons for both the observations.
Ans. This is due to forces of attraction among particles of water and later between particles of water and particles of the floor.
Q. No. 4) Which of the following are matter?
Chair, air, love, smell, hate, almonds, thought, cold, lemon water, the smell of perfume.
Ans. Chair, air, almonds, lemon water.
Q. No. 5) What are the characteristics of the particles of matter?
Ans. i. Particles of matter have space between them.
ii. Particles of matter are continuously moving.
iii. Particles of matter attract each other.
iv. Particles of matter are very small.
Q. No. 6) Name the temperature at which solid and liquid states of matter coexist.
Ans. Melting point
Q. No. 7) Why is the density of solids higher than gases?
Ans. Particles of solids are tightly packed as compared to particles of gases as the forces of attraction between particles of solids are more than gases.
Q. No. 8) Give reason: The smell of hot sizzling food reaches you several meters away, but to get the smell of cold food you have to go close.
Ans. Particles of the aroma of hot food have more kinetic energy compared to that of cold food. Due to more kinetic energy, they are able to diffuse more quickly in air and reach our noses.
Q.No.9) Tabulate the differences in the characteristics of states of matter.
Ans.
| Characteristics | Solid | Liquid | Gas |
| 1. Shape | fixed shape | no fixed shape | no fixed shape |
| 2. Volume | fixed volume | fixed volume | no fixed volume |
| 3. Rigidity/fluidity | rigid, cannot flow | not rigid, can flow | not rigid, can flow |
| 4. Intermolecular force | maximum | less than solids | very less |
| 5. Intermolecular space | very less | more than solids | maximum |
| 6. Compressibility | negligible | incompressible | highly compressible |
Q. No. 10) Why does diffusion become faster on heating?
Ans. As the temperature increases, the kinetic energy of molecules increases. This increases the intermolecular space between particles because of which the matter can diffuse more easily into other matter.
Q. No. 11) How does evaporation cause a cooling effect?
Ans. The particles of liquid absorb energy from the surroundings to regain the energy lost during evaporation. This absorption of energy from the surroundings makes the surroundings cold.
Q.No.12) Comment on the following statements:
a) Desert cooler cools better on a hot dry day.
b) Water kept in an earthen pot (matka) become cool during summer.
c) Doctors advise putting strips of wet cloth on the forehead of a person having a high temperature.
Ans. a) In the outer wall of the desert cooler, the water gets continuously sprinkled. Due to the dry weather outside, this water gets evaporated at a high rate. During the evaporation process, the heat inside the cooler, the air passing through the interior of the cooler gets cooled down and is pushed into the room by a fan.
b) The earthen pot naturally is porous in structure. The water stored in it penetrates through the walls and at the outer surface gets evaporated. During the evaporation process, the surrounding surface that is the inner surface gets cooled down as the heat from the adjacent layer is used for evaporation. This process cools the inner surface of the earthen pot and the water inside the pot loses its heat through the same process.
c) Evaporation of water from wet strip requires heat which is taken from This will lower the body temperature.
Q.No.13) Rahul wants to wear his favorite shirt to a party, but the problem is that it is still wet after a wash. What steps would you suggest to dry it faster?
Ans. Conditions that can increase the rate of evaporation of water are:
i. An increase of surface area by spreading the shirt.
ii. An increase in temperature by putting the shirt under the sun.
iii. Increase the wind speed by spreading it under the fan.
Q. No. 14) The image shows three substances that can change from one physical state to another by different processes.
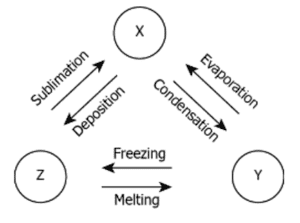
What are X, Y, and Z?
Ans. X- Gas, Y- Liquid, Z - Solid
Q. No. 15) Why does the temperature remain constant during the melting of a solid/boiling of a liquid?
Ans. The temperature of a substance remains constant at its melting and boiling points until all of the substance melts or boils because the heat supplied is continuously used up in changing the state of the substance by overcoming the forces of attraction between the particles. This heat energy absorbed without showing any rise in temperature is given the name latent heat of fusion/latent heat of vaporization.
Q. No. 16) Define
i. Latent heat of fusion
ii. Latent heat of vaporization
Ans. i. The amount of heat energy that is required to change 1 kg of a solid into liquid at atmospheric pressure at its melting point is known as the latent heat of fusion.
ii. The amount of heat energy that is required to change 1 kg of a liquid into a gas at atmospheric pressure at its boiling point is known as the latent heat of vaporization.
Q. No. 17) Fill in the blanks:
a. At room temperature the forces of attraction between the particles of solid substances are _________ than those which exist in the gaseous state.
b. The arrangement of particles is less ordered in the ________ state. However, there is no order in the ______ state.
c._______ is the change of gas state directly to solid state without going through the _________ state.
d. The phenomenon of change of a liquid into the gaseous state at any temperature below its boiling point is called ________.
Ans. a. stronger
b. liquid, gaseous
c. Deposition
d. Evaporation.
Q.No. 18) Match the physical quantities given in column A to their SI units given in column B:
a. Pressure i. cubic metre
b. Temperature ii. Kilogram
c. Density iii. Pascal
d. Mass iv. Kelvin
e. Volume v. kilogram per cubic metre
Ans. a-iii, b-iv, c-v, d-ii, e-i
Also Read: |
Watch Full Chapter 1 Explanation of Matter in Our Surroundings Here:
no-1… +223 hoga