In CBSE Class 9 Science, Chapter 4, Structure of the Atom, plays a crucial role in building a foundation for understanding atomic theory and the fundamental particles of matter. This chapter introduces students to key concepts such as atoms, subatomic particles (protons, neutrons, and electrons), and their respective properties. As you prepare for exams, it's essential to be well-versed in the important questions and answers related to this chapter. This post aims to guide you through the critical topics, offering concise explanations and solutions to help reinforce your understanding and boost your confidence for the upcoming tests.
IMPORTANT QUESTIONS & ANSWERS
1. What are canal rays?
Ans: Positively charged radiations discovered by E. Goldstein in a gas discharge tube.
2. If an atom contains one proton and one electron, will it carry any charge?
Ans: No, because the +1 charge of the proton and –1 charge of the electron cancel each other.
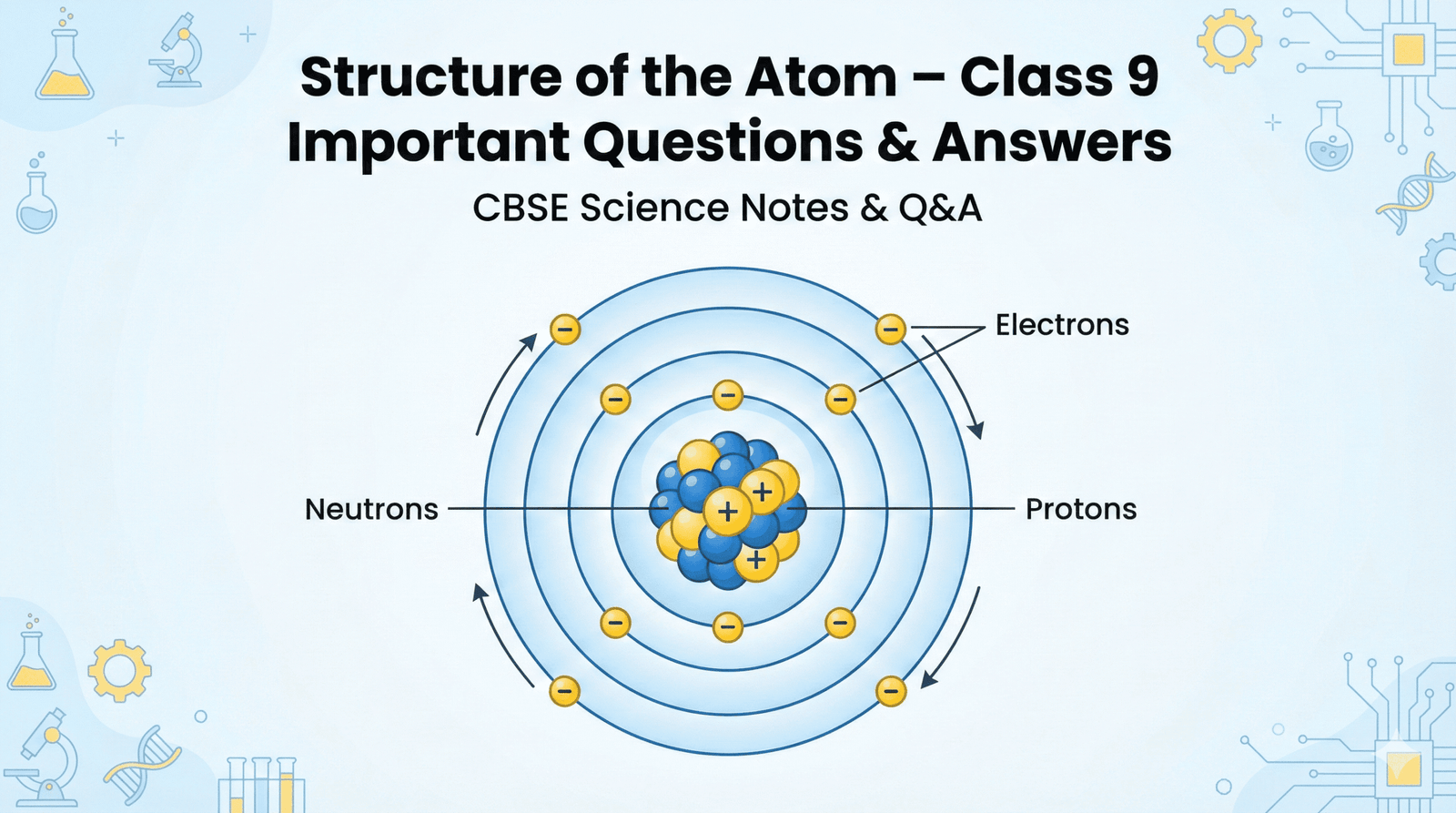
3. Name the three sub-atomic particles of an atom.
Ans: Electron, proton, neutron.
4. Compare the properties of electrons, protons and neutrons.
Ans.
| Property | Electron (e⁻) | Proton (p⁺) | Neutron (n) |
|---|---|---|---|
| Charge | Negative (-1) | Positive (+1) | Neutral (0) |
| Mass | Negligible (~1/2000 u) | 1 u | 1 u |
| Location in Atom | Outside nucleus (in shells) | Nucleus | Nucleus |
| Discovery | J.J. Thomson | E. Goldstein | J. Chadwick |
5. State Thomson’s model of atom and its two limitations.
Ans: Thomson proposed that:
- (i) An atom consists of a positively charged sphere and the electrons are embedded in it.
- (ii) The negative and positive charges are equal in magnitude. So, the atom as a whole is electrically neutral.
Limitations of Thomson's Model of Atom:
- It could not explain the results of Rutherford’s scattering experiment.
- It failed to explain the arrangement of electrons.
6. Describe Rutherford’s α-particle scattering experiment. State observations, conclusions, and how it proved the existence of the nucleus.
Answer:
Experiment:
- A thin gold foil (~1000 atoms thick) was bombarded with fast-moving α-particles.
- A fluorescent zinc sulphide screen detected deflections.
Observations:
- Most α-particles passed straight through.
- Some were deflected at small angles.
- A very few (1 in 12000) rebounded.
Conclusions:
- Most of the space in an atom is empty.
- Positive charge and most of the mass are concentrated in a very small region called the nucleus.
- Electrons revolve around the nucleus like planets around the sun.
- Size of nucleus is 10⁵ times smaller than the atom.
This experiment proved the existence of a small, dense, positively charged nucleus.
7. Explain Rutherford’s nuclear model of an atom.
Ans: Rutherford proposed:
- Atom has a small, dense, positively charged nucleus.
- Nearly all mass is in the nucleus.
- Electrons revolve around the nucleus in circular paths.
- Most of the atom is empty space.
8. Explain drawbacks of Rutherford’s model.
Ans:
- According to electromagnetic theory, accelerating electrons should lose energy and fall into nucleus.
- It could not explain the stability of atom.
9. What would happen if Rutherford performed his scattering experiment using a metal foil other than gold?
Ans:
The same general observations would be obtained because the structure of atoms is similar for all elements.
However, deflections may differ slightly depending on the atomic number of the metal.
10. Describe Bohr’s model of the atom. How does it explain stability of atoms?
Ans: Bohr proposed:
- Electrons revolve in fixed orbits of definite energy levels.
- Electrons do not radiate energy in these orbits.
- Energy is absorbed/emitted only when electrons jump orbits.
Explanation of stability:
Since electrons do not radiate energy while revolving in fixed orbits, they do not fall into the nucleus, making atoms stable.
11. Write the rules for distributing electrons into different shells (Bohr–Bury scheme). Explain with an example.
Answer: Rules:
- Maximum electrons in a shell = 2n²
- K(1) = 2
- L(2) = 8
- M(3) = 18
- Outermost shell cannot have more than 8 electrons.
- A new shell cannot start filling until the inner one is completely filled.
Example:
Sodium (Z = 11):
K = 2
L = 8
M = 1
→ Electron distribution = 2, 8, 1
Note: Important Table to Remember:
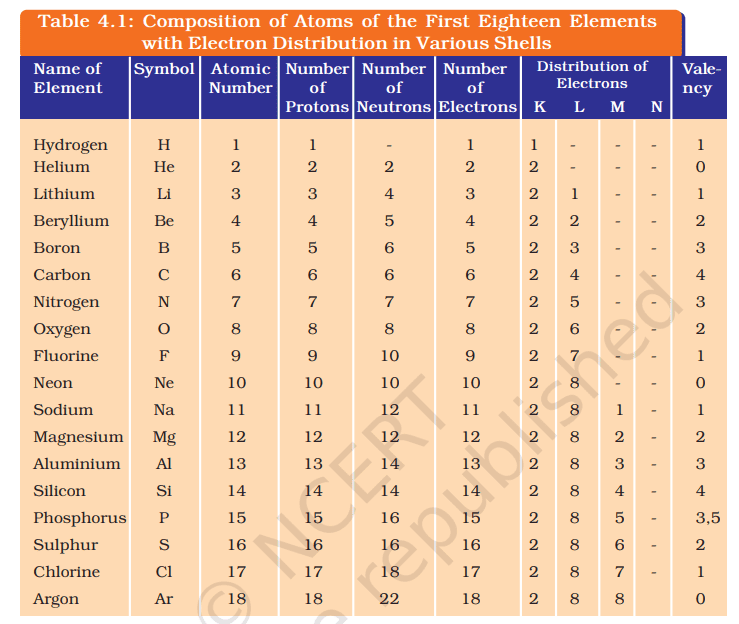
12. If K and L shells are completely filled, how many electrons are there in the atom?
Ans:
K-shell = 2 electrons
L-shell = 8 electrons
Total = 10 electrons
13. Write the electron distribution of carbon and sodium.
Ans:
- Sodium (Z=11) → 2, 8, 1
- Carbon (Z=6) → 2, 4
14. Explain valency. Describe how valency is determined using the examples of oxygen and aluminium.
Answer: Valency: The combining capacity of an atom determined by the number of electrons lost, gained, or shared to attain a full outer shell.
Oxygen:
- Has 6 electrons in outer shell
- Needs 2 more to complete octet
→ Valency = 2
Aluminium:
- Has 3 electrons in outer shell
- Loses 3 electrons
→ Valency = 3
15. An atom has 8 electrons and 8 protons. (i) What is its atomic number? (ii) What is its charge?
Ans:
(i) Atomic number = 8
(ii) Charge = neutral (8+ and 8− cancel out)
16. Define atomic number, mass number, isotopes and isobars with examples. Write any two applications of isotopes.
Ans.
- 1. Atomic Number (Z): The atomic number of an element is the number of protons present in the nucleus of an atom of that element. Example: Atomic number of Carbon = 6 (so it has 6 protons).
- 2. Mass Number (A): The mass number of an atom is the sum of protons and neutrons in its nucleus. Example: Mass number of Carbon-12 = 12 (6 protons + 6 neutrons).
- 3. Isotopes: Isotopes are atoms of the same element having the same number of protons but different numbers of neutrons, and hence different mass numbers. Example: Carbon-12 and Carbon-14 are isotopes of Carbon.
Applications of Isotopes:
- Used in medicine for diagnosis and treatment (e.g., Cobalt-60 in cancer therapy).
- Used in agriculture to improve crop varieties and study fertilizers.
- Used in industry to detect leaks and study wear and tear in machines.
- 4. Isobars: Isobars are atoms of different elements having the same mass number but different atomic numbers. Example: Carbon-14 (6 protons + 8 neutrons) and Nitrogen-14 (7 protons + 7 neutrons) are isobars.
17. Calculate the average atomic mass of bromine if two isotopes occur as:
79Br (49.7%), 81Br (50.3%)
Ans:
= (79 × 0.497) + (81 × 0.503)
= 39.263 + 40.743
= 80.006 u ≈ 80 u
18. A sample of element X has an average atomic mass of 16.2 u. It contains isotopes ¹⁶X and ¹⁸X. Find their percentage.
Ans. Let % of ¹⁸X = y
Then % of ¹⁶X = 100 – y
16(100–y)/100 + 18y/100 = 16.2
Solve: y = 10%
Therefore, ¹⁶X → 90% and ¹⁸X → 10%
Relevant Articles:
- CBSE Class 9 Sound Chapter Important Questions for Exams (NCERT Focused)
- CBSE Class 9 Science Chapter 10 Work and Energy Important Questions & Answers
- Improvement in Food Resources: CBSE Class 9 Science Important Questions and Answers
- Structure of the Atom – Important Questions and Answers for CBSE Class 9
- Atoms and Molecules Class 9 – Most Important Questions and Answers