Undoubtedly, understanding Acids Bases and Salts can be a tricky task. Fortunately, we have compiled an extensive set of questions and answers related to Class 10 Science Chapter 2 Acids, Bases, and Salts. These questions are sourced from CBSE Previous Year Questions, CBSE Official Sample Papers, and NCERT Exemplar among other sources to help you better understand the topic.

| Subject | Science (Chemistry) |
| Class | 10 |
| Board | CBSE & State Boards |
| Chapter No. | 2 |
| Chapter Name | Acids, Bases, and Salts |
| Type | Important Questions & Answers |
| Session | 2024-25 |
"The only limit to our realization of tomorrow will be our doubts of today."
- Franklin D. Roosevelt
Acids, Bases, and Salts Class 10 Important Questions with Answers
Q. No. 1) Multiple Choice Questions (MCQs):
i. Meena rubbed the yellow-colored turmeric stain on his son’s shirt with soap. She observed the color of the stain became:
a. Pink
b. Reddish brown
c. Remained yellow
d. White
Ans. Option (b).
ii. The yellow color of turmeric changes to red on addition of soap solution. When substance P is added to turmeric, there is no change in color.
Which of the following is definitely true about substance P?
a. P is an acid
b. P is not a salt
c. P is not a base
d. P is a neutral substance
Ans. Option (c)
iii. Which of the following are properties of acids?
- They are bitter in taste.
- They react with metals to produce hydrogen gas.
- They are easily soluble in water.
Options
a. Only P
b. Only P and R
c. Only Q and R
d. All – P, Q, and R
Ans. Option (c).
iv. Anand took four colorless solutions P, Q, R, and S, and performed the following tests.
| Solution P | Solution Q | Solution R | Solution S | |
| With Methyl Orange | No change in color | Turns red | No change in color | No change in color |
| With Phenolphthalein | No change in color | No change in color | No change in color | Turns pink |
| With Red litmus | No change in color | No change in color | No change in color | Turns litmus blue |
| With Blue litmus | No change in color | Turns litmus red | No change in color | No change in color |
What is the definite conclusion that Anand can reach?
a. Both P and S are salt solutions
b. Both Q and S are basic solutions
c. Both Q and R are salt solutions
d. Both P and R are neutral solutions.
Ans. Option (d).
v. In a very polluted city, water from the first rain in the monsoon season is collected in a beaker. Which of the following changes will it have on a litmus paper?
a. Turn red litmus blue.
b. It will have no effect on the litmus paper.
c. Both A and B
d. Turn blue litmus red
Ans. Option (d).
vi. Which of the following reactions is a neutralization reaction?
a. 4Na + O2 → 2Na2O
b. Fe + 2HCl → FeCl2 + H2
c. MgO + H2O → Mg(OH)2
d. HNO3 + NaOH → NaNO3 + H2O
Ans. Option (d).
vii. In an attempt to demonstrate electrical conductivity through an electrolyte, the following apparatus was set up.
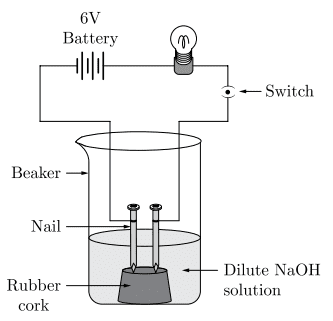
Which among the following statement(s) is/are correct?
- The bulb will not glow because the electrolyte is not acidic.
- The bulb will glow because NaOH is a strong base and furnishes ions for conduction.
- The bulb will not glow because the circuit is incomplete.
- The bulb will not glow because it depends upon the type of electrolytic solution.
a. 1 and 3
b. 2 only
c. 2 and 4
d. 4 only
Ans. Option (b).
viii. Which of the following are correctly matched?
| 1. Acid + Salt | Metal + Hydrogen |
| 2. Acid + Metal Carbonate | Salt + Carbon Dioxide + Water |
| 3. Metal Oxide + Acid | Salt + Water |
Options:
a. 1 and 2
b. 2 and 3
c. 1 and 3
d. 1, 2 and 3
Ans. Option (b).
ix. Which of the following are correctly matched?
| 1. Common salt | Formed by sodium hydroxide and hydrochloric acid |
| 2. Brine | The aqueous solution of Sodium chloride |
| 3. Chlor-alkali process | Formation of Sodium chloride |
a. 1 and 2
b. 2 and 3
c. 1 and 3
d. 1, 2 and 3
Ans. Option (a).
x. Payal has to arrange the following in decreasing order of hydroxide ion concentration.
Wine (pH 4.0), Milk of magnesia (pH 10.5), Blood (pH 7.4)
Which of the following arrangements is correct?
a. Wine → Milk of magnesia → Blood
b. Blood → Milk of magnesia → Wine
c. Milk of Magnesia → Blood → Wine
d. Wine → Blood → Milk of Magnesia
Ans. Option (c).
xi. Anita added a drop each of diluted acetic acid and diluted hydrochloric acid on pH paper and compared the colors. Which of the following is the correct conclusion?
a. pH of acetic acid is more than that of hydrochloric acid.
b. pH of acetic acid is less than that of hydrochloric acid.
c. Acetic acid dissociates completely in an aqueous solution.
d. Acetic acid is a strong acid.
Ans. Option (a).
xii. The graph given below depicts a neutralization reaction (acid + base → salt + water). The pH of a solution changes as we add an excess of acid to an alkali.
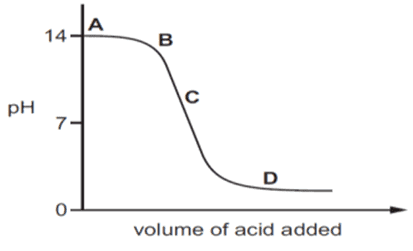
Which letter denotes the area of the graph where both acid and salt are present?
a. A
b. B
c. C
d. D
Ans. Option (d).
xiii. A solution of a base with pH 12.1 is given.
Which of the following can be done to decrease its pH?
- Add distilled water to it.
- Add a solution of a different base with pH 8.7
- Add a few drops of an acid with an unknown pH
Options
a. Only 1
b. Only 3
c. Only 1 and 2
d. Any of 1, 2, and 3
Ans. Option (d)
xiv. Some activities cause the soil and water resources in that area to become acidic. Once these activities are stopped, the land has to be treated to enable plants to grow once again.
Which of the following should be added to the land to decrease the acidity permanently and allow plants to grow once again?
a. Water which is neutral.
b. Calcium oxide which is basic.
c. Sodium chloride which is neutral.
d. Dilute hydrochloric acid solution.
Ans. Option (b).
xv. Identify the basic salt from the following salts:
a. Na2CO3
b. NH4Cl
c. NaNO3
d. KCl
Ans. Option (a).
xvi. Mild non-corrosive basic salt is
a. Ca(OH)2
b. NaCl
c. NaOH
d. NaHCO3
Ans. Option (d)
xvii. Which of the following salts does not contain water of crystallization?
a. Blue vitriol
b. Baking soda
c. Washing soda
d. Gypsum
Ans. Option (b).
xviii. Match the chemical substances given in column A with their appropriate application given in column B.
| Column A | Column B |
| A. Bleaching powder | i. Preparation of glass |
| B. Baking Soda | ii. Production of H2 and Cl2 |
| C. Washing Soda | iii. Decolorization |
| D. Sodium Chloride | iv. Antacid |
a. A-ii, B-i, C-iv, D-iii
b. A-iii, B-ii, C-iv, D-i
c. A-iii, B-iv, C-i, D-ii
d. A-ii, B-iv, C-i, D-iii
Ans. Option (c).
xix. Which of the following are correctly matched?
| 1. Plants and animals | pH range is 7.0 to 7.8 |
| 2. Acid rain | pH is 7.6 |
| 3. Tooth Decay | pH less than 5.5 |
a. 1 and 2
b. 2 and 3
c. 1 and 3
d. 1, 2, and 3
Ans. Option (c). (pH of acid rain is less than 5.6)
Q. No. 2) Assertion Reason-Based Questions:
i. For the given question numbers, two statements are given, one labeled Assertion (A) and the other labeled Reason (R). Select the correct answer from the given options.
Assertion (A): While dissolving an acid in the water, the water must always be added slowly to the acid with constant stirring.
Reason (R): Dissolving an acid in water is a highly exothermic reaction.
a. Both A and R are true and R is the correct explanation of the assertion.
b. Both A and R are true and R is not the correct explanation of the assertion.
c. A is true but R is false
d. A is false but R is true
Ans. Option (d).
ii. Assertion (A): Fresh milk in which baking soda is added, takes a longer time to set as curd.
Reason (R): Baking soda decreases the pH value of fresh milk to below 6.
a. Both A and R are true and R is the correct explanation of the assertion.
b. Both A and R are true and R is not the correct explanation of the assertion.
c. A is true but R is false
d. A is false but R is true
Ans. Option (c).
Q. No. 3) The reaction between MnO2 with HCl is depicted in the following diagram. It was observed that a gas with bleaching abilities was released.
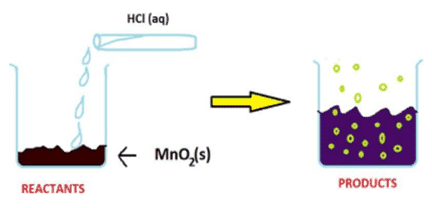
i. The chemical reaction between MnO2 and HCl is an example of:
a. Displacement reaction
b. Combination reaction
c. Redox reaction
d. Decomposition reaction
Ans. Option (c).
ii. Chlorine gas reacts with _____ to form bleaching powder.
a. Dry Ca(OH)2
b. Dil. Solution of Ca(OH)2
c. Conc. Solution of Ca(OH)2
d. Dry CaO
Ans. Option (a)
iii. Identify the correct statement from the following:
a. MnO2 is getting reduced whereas HCl is getting oxidized.
b. MnO2 is getting oxidized whereas HCl is getting reduced.
c. MnO2 and HCl both are getting reduced.
d. MnO2 and HCl both are getting oxidized.
Ans. Option (a)
iv. In the above-discussed reaction, what is the nature of MnO2?
a. Acidic oxide
b. Basic oxide
c. Neutral oxide
d. Amphoteric oxide
Ans. Option (b)
v. What will happen if we take dry HCl gas instead of an aqueous solution of HCl?
a. Reaction will occur faster.
b. Reaction will not occur.
c. Reaction rate will be slow.
d. Reaction rate will remain the same.
Ans. Option (b).
Q. No. 4) Write the chemical name and chemical formula of the salt used to remove the permanent hardness of the water.
Ans. Chemical Name: Sodium Carbonate Decahydrate (Washing Soda)
Chemical Formula: Na2CO3.10H2O
Q. No. 5) What do you understand by olfactory indicators?
Ans. Some indicators show a change in their odor in the presence of acids or bases. Such indicators are called olfactory indicators. They are very useful for visually challenged students because such students cannot use other indicators.
Clove, vanilla, and onion are examples of olfactory indicators.
Q. No. 6) Dil. HCl is added to Zn granules.” How will you prove that chemical change has taken place here? Support your response with two arguments.
Ans.
- Bubbles of gas/ Evolution of gas
- Change in color (Zn - silvery grey to black)
- Change in temperature
Q. No. 7) In the following schematic diagram for the preparation of hydrogen gas as shown in the figure, what would happen if the following changes are made?
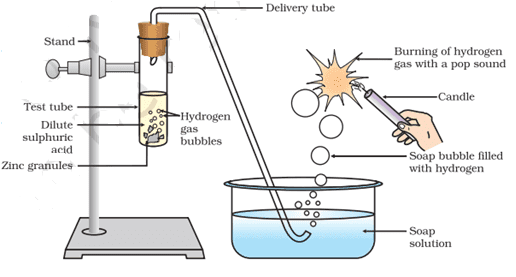
a. In place of Zn granules, the same amount of zinc dust is taken in the test tube.
b. Instead of dilute sulphuric acid, dilute hydrochloric acid is taken.
c. In place of Zn, Cu turnings are taken.
d. Sodium hydroxide is taken in place of sulphuric acid and the tube is heated.
Ans. a. Hydrogen gas will evolve with a greater speed. (Because Dust particles are smaller in comparison to granules, hence increase the surface area of the reaction)
b. Almost the same amount of gas is evolved. (Both are strong acids).
c. Hydrogen gas is not evolved. (Because no reaction will take place as in the metal reactivity series, Cu is below hydrogen, and it cannot displace hydrogen from acids.)
d. If sodium hydroxide is taken, hydrogen gas will be evolved.
Zn + 2NaOH → Na2ZnO2 (Sodium Zincate) + H2.
Q. No. 8) Varun took sample A and added dilute hydrochloric acid to it. A colorless, odorless gas X was evolved which turned lime water milky. Identify sample A and the gas X evolved. Write a chemical equation to explain the reaction between sample A and Hydrochloric acid. Why does the gas X turn lime water milky?
Ans. Sample A is metal carbonate and the gas X is carbon dioxide.
Na2CO3 + 2HCl → 2NaCl + CO2 + H2O
CO2 reacts with lime water to form insoluble calcium carbonate which is white in color and results in milkiness.
Ca(OH)2 + CO2 → CaCO3 + H2O
Q. No. 9) What is a neutralization reaction?
Ans. A neutralization reaction is a reaction between an acid and a base to form salt and water. It is an exothermic reaction.
Eg. HCl + NaOH → NaCl + H2O
Q. No. 10) List two differences between acids and bases on the basis of chemical properties.
Ans.
| Acids | Bases |
| 1. Acids turn blue litmus red. | 1. Bases turn red litmus blue. |
| 2. Acids liberates CO2 with metal carbonates and hydrogen carbonates. | 2. Bases do not react with metal carbonates and hydrogen carbonates. |
Q. No. 11) Differentiate between acidic and basic salt with an example of each case.
Ans. Acidic salts are formed by the neutralization reaction between a strong acid and a weak base. Eg. Ammonium chloride. Whereas, basic salts are formed by the neutralization reaction between a strong base and a weak acid. Eg. Sodium acetate.
Q. No. 12) Fill in the missing data in the following table:
| Name of the Salt | Formula | Salt obtained from | |
| Acid | Base | ||
| i. Ammonium Chloride | NH4Cl | NH4OH | - |
| ii. Copper Sulphate | - | - | H2SO4 |
| iii. Sodium Chloride | NaCl | NaOH | - |
| iv. Magnesium nitrate | Mg(NO3)2 | - | HNO3 |
| v. Potassium sulphate | K2SO4 | - | - |
| vi. Calcium nitrate | Ca(NO3)2 | Ca(OH)2 | - |
Ans.
| Name of the Salt | Formula | Salt obtained from | |
| Acid | Base | ||
| i. Ammonium Chloride | NH4Cl | NH4OH | HCl |
| ii. Copper Sulphate | CuSO4 | Cu(OH)2 | H2SO4 |
| iii. Sodium Chloride | NaCl | NaOH | HCl |
| iv. Magnesium nitrate | Mg(NO3)2 | Mg(OH)2 | HNO3 |
| v. Potassium sulphate | K2SO4 | KOH | H2SO4 |
| vi. Calcium nitrate | Ca(NO3)2 | Ca(OH)2 | HNO3 |
Q. No. 13) You are provided with 90 mL of distilled water and 10 mL of concentrated sulphuric acid to prepare dilute sulphuric acid.
i. What is the correct way of preparing dilute sulphuric acid? Give reason.
ii. How will the concentration of H3O+ ions change on dilution?
Ans. i. Add 10 mL of concentrated sulphuric acid slowly to 90 mL of water with constant stirring.
Dilution of acid is a highly exothermic process. If water is added to concentrated sulphuric acid, the heat generated causes the mixture to splash leading to burns and the glass container can break.
ii. Decreses per unit volume.
Q. No. 14) Give suitable reasons for the following statements:
i. Rainwater conducts electricity but distilled water does not.
ii. We feel a burning sensation in the stomach when we overeat.
iii. A tarnished copper vessel regains its shine when rubbed with lemon.
iv. The crystals of washing soda change to white powder on exposure to air.
v. An aqueous solution of sodium chloride is neutral but an aqueous solution of sodium carbonate is basic.
vi. Dry hydrogen chloride gas does not turn blue litmus red whereas dilute hydrochloric acid does.
vii. During the summer season, a milkman usually adds a very small amount of baking soda to fresh milk.
viii. Ammonia is a base but it does not contain a hydroxyl group.
Ans. i. Distilled water is a pure form of water. It is neither acidic nor basic in nature. So distilled water does not dissociate into ions, since, the conduction of electricity requires free ions so, distilled water does not conduct electricity. While rainwater being an impure form of water contains many ionic species. These ions are responsible for the electrical conductivity of rainwater.
ii. We feel a burning sensation in the stomach when we overeat because when we overeat too much HCl is produced in our stomach which causes the burning sensation.
iii. Copper vessels tarnish due to the formation of basic copper carbonate which gets neutralized when rubbed with lemon and the copper vessel regains its shine.
iv. Washing soda (Na2CO3.10H2O) when exposed to air, it loses 10 molecules of water and changes to white powder.
v. Sodium chloride is a salt of strong acid HCl and a strong base NaOH, so it is neutral. Sodium carbonate is a salt of weak acid H2CO3 and strong base NaOH, so it is basic.
NaCl + H2O → NaOH + HCl
Na2CO3 + H2O → NaOH + H2CO3
vi. Dry HCl gas does not dissociate into ions (H3O+), so it has no effect on the litmus. Hydrochloric acid forms ions (H3O+), so it turns blue litmus red.
vii. Baking soda prevents the formation of lactic acid when milk turns sour.
viii. Ammonia (NH3) dissolves in H2O forming NH4OH, therefore it acts as base:
NH3 + H2O → NH4OH → NH4+ + OH-
Q. No. 15) i. Give the chemical name of the compound present in tooth enamel. What is the nature of the compound?
ii. ‘Sweet tooth may lead to tooth decay.’ Explain why? What is the role of toothpaste in preventing cavities?
Ans. i. Chemical Name: Calcium Phosphate
Nature: Basic.
ii. Sweet tooth leads to tooth decay which is caused by the action of bacteria on food particles remaining in the mouth and acid is formed. The pH of the mouth falls below 5.5 and the tooth enamel dissolves resulting in cavities. Toothpaste is generally basic, they neutralize the excess acid produced in the mouth and prevent tooth decay.
Q. No. 16) Why does a honey bee sting cause pain and irritation? Give a method to get relief from the discomfort.
Ans. The bee sting leaves an acid called formic acid in the body. Relief can be obtained by rubbing a mild base such as toothpaste or sodium bicarbonate (baking soda) on the affected area, as it is a mild base and neutralizes the effect of formic acid.
Q. No. 17) Name one natural source of each of the following:
i. Citric acid
ii. Oxalic acid
iii. Acetic acid
iv. Lactic acid
v. Methanoic acid
vi. Tartaric acid
Ans. i. Citrus fruits like lemon, orange
ii. Tomato/Spinach
iii. Vinegar
iv. Sour milk (curd)
v. Ant sting/nettle sting
vi. Tamarind
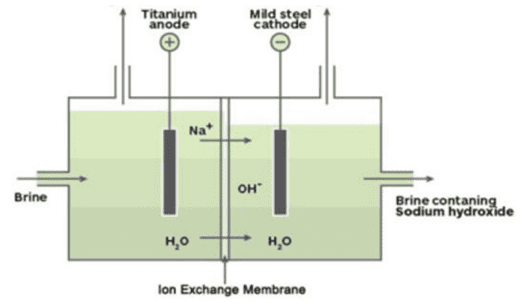
Q. No. 18) a. Identify the gases evolved at the anode and cathode in the below experimental setup.
b. Name the process that occurs. Why is it called so?
c. Illustrate the reaction of the process with the help of a chemical equation.
d. Write one use of each of the products of the above process. Write examples of other compounds that can be formed using Sodium chloride as a raw material (reactant).
Ans. a. Anode: Chlorine; Cathode: Hydrogen.
b. Chlor alkali process as the products obtained are alkali, chlorine gas, and hydrogen gas.
c. 2NaCl + 2H2O + electric current → 2NaOH + Cl2 + H2
d. Sodium hydroxide is used in the making of soaps and detergents, paper making.
Chlorine is used as a disinfectant (water treatment, swimming pools), and as an oxidizing agent.
Hydrogen is used as fuel, making ammonia for fertilizers.
Baking soda and Washing soda can be prepared by using sodium chloride as a raw material (reactant).
Q. No. 19) A metal carbonate X reacting with acid gives a gas which when passed through a solution Y gives the carbonate back. On the other hand, a gas G that is obtained at the anode during electrolysis of brine is passed on dry Y, it gives a compound Z, used for disinfecting drinking water. Identify X, Y, G, and Z. Also write the chemical reactions involved.
Ans. The gas G that evolved at the anode during the electrolysis of brine is chlorine.
When chlorine gas is passed through dry Ca(OH)2 (Y), it produces bleaching powder (CaOCl2) (Z) used for disinfecting drinking water.
Ca(OH)2 + Cl2 → CaOCl2 + H2O
Since Y and Z are calcium salts, therefore X is also a calcium salt and is calcium carbonate.
CaCO3 + 2HCl → CaCl2 + CO2 + H2O
Ca(OH)2 + CO2 → CaCO3 + H2O
Q. No. 20) i. Match the following:
| Milk of Magnesia | 1 |
| Gastric Juices | 7 |
| Brine | 10 |
| Aqueous Sodium Hydroxide | 13 |
ii. Amit and Rita decided to bake a cake and added baking soda to the cake batter.
Explain with a balanced reaction, the role of the baking soda. Mention any other use of baking soda.
Ans. i. Milk of magnesia – 10
Gastric juices – 1
Brine – 7
Aqueous sodium hydroxide – 13
ii. Baking soda undergoes thermal decomposition to form Na2CO3, CO2, and H2O. CO2 makes the cake fluffy & soft.
NaHCO3 → Na2CO3 + CO2 + H2O
Other uses:
- Used in fire extinguishers
- Antacid to neutralize excess acid in the stomach
- To neutralize the effect of acid in insect stings.
Q. No. 21) Chlorine gas was prepared using electrolysis of brine solution. Write the chemical equation to represent the change. Identify the other products formed in the process and give one application of each.
Ans. 2NaCl (aq) + 2H2O (l) → 2NaOH (aq) + Cl2 (g) + H2 (g)
Other products formed during the electrolysis of brine solution are:
- Sodium hydroxide/ NaOH/ Caustic soda
- Hydrogen
Uses:
1. Sodium hydroxide/NaOH/Caustic soda
- Degreasing of metals
- Preparation of soaps and detergents
- Papermaking
- Artificial fibers
2. Hydrogen
- Fuels
- Margarine
- Manufacture of ammonia for fertilizers
Q. No. 22) i. Write the name and chemical formula of the calcium compound used as a disinfectant. How is this compound manufactured?
ii. Name the products obtained when sodium hydrogen carbonate is heated. Write the chemical equation for the reaction.
Ans. i. Name: Bleaching Powder
Chemical formula: CaOCl2
Preparation: On passing chlorine gas through slaked lime bleaching powder is formed.
Ca(OH)2 + Cl2 → CaOCl2 + H2O
ii. The products obtained are sodium carbonate, carbon dioxide, and water.
2NaHCO3 → Na2CO3 + CO2 + H2O
Q. No. 23) Diana prepared a cake by two methods.
Method (i): She added baking soda to the cake mixture and let the mixture stand for one hour before placing it in the oven to bake.
Method (ii): She added baking powder to the cake mixture and let the mixture stand for one hour before placing it in the oven to bake.
State the difference in the cake mixtures that Diana is likely to have observed before baking. Explain why.
Ans. Diana is likely to see that the cake mixture (ii) has risen while cake mixture (i) has not.
The sodium bicarbonate and tartaric acid in baking powder react on mixing with one another, producing carbon dioxide that causes the cake mixture to rise.
NaHCO3 + H+(from tartaric acid) → CO2 + H2O + Sodium salt of acid
Baking soda does not contain tartaric acid and hence does not produce carbon dioxide before baking.
Q. No. 24) What is meant by the term water of crystallization? How would you show that copper sulphate crystals contain water of crystallization?
Ans. The molecules of water associated with a crystalline substance are called water of crystallization.
When hydrated copper sulphate is heated its color changes from blue to dirty white and water droplets are formed.
CuSO4.5H2O → CuSO4 + 5H2O.
If we add little water to anhydrous CuSO4, we get blue color again. It is the presence of molecules of water of crystallization that was lost on heating.
CuSO4 + 5H2O → CuSO4.5H2O
Q. No. 25) i. Rahul found that the Plaster of Paris, which he stored in a container, has become very hard and lost its binding nature. What is the reason for this? Also, write a chemical equation to represent the reaction taking place.
ii. Give any one use of Plaster of Paris other than for plastering or smoothening of walls.
Ans. i. Due to moisture in the atmosphere, it converted into Gypsum.
CaSO4.1⁄2H2O + 11⁄2H2O → CaSO4.2H2O (Gypsum)
ii. Uses of Plaster of Paris:
- Making toys, dolls, or statues
- Fixing broken limbs
- Making decorative materials.
Q. No. 26) Identify the compound of calcium that is used for plastering fractured bones. With the help of a chemical equation show how is it prepared and what special precautions should be taken during the preparation of this compound.
Ans. Plaster of Paris (CaSO4.1⁄2H2O)
On heating gypsum at 373 K, it loses water molecules and becomes calcium sulphate hemihydrate
CaSO4.2H2O (Heat at 373K)→ CaSO4.1⁄2H2O + 11⁄2H2O
Precaution: Gypsum should not be heated above 373K otherwise it will form CaSO4.
| Must Read: Acids, Bases, and Salts Class 10 Notes to get an idea of the different types of questions asked from this chapter. |
| You Might Also Like: CBSE Class 10 Notes CBSE Class 10 Important Questions and Answers |
Hope you liked these important questions and answers on Class 10 Chemistry Chapter 2 Acids, Bases, and Salts. Please share this with your friends and do comment if you have any doubts/suggestions to share.
Frequently Asked Questions (FAQs):
What is the name of the reaction between acid and base class 10?
Neutralization reaction: In this reaction, an acid, and a base react with each other and form salt and water.
Eg: HCl + NaOH → NaCl + H2O
What acid is present in the stomach?
Hydrochloric acid (HCl)
What are 3 common acids?
Sulphuric acid, hydrochloric acid, nitric acid.
Sir English ka important question kaise likha
Please help me sir 👑
English ke liye hum Line by line Chapter explain kar chuke hai. Aap usko prepare kijiye kyuki isme inside text se questions aata hai.
Waiting for this thank you sir 😊
You are most welcome 🙂
Thanks a BILLION for this website, your channel and eventually you sir..udk how much u helped me to get good marks especially in science..I used to be so weak in sci..maybe becoz I hated it, but now I luv it! All becoz of u!! Thank you sm again~
Thank you so much for this lovely comment 😊
You’re most welcome sir✨
Sir will this type of questions come in boards…. Cbse
Yes these questions are compiled from CBSE official sample papers and pyqs. You must prepare these questions.
Thank you so much sir 💕
Sir I have doubt in ix ( 9 MCQ)
Common salt NaOH and HCL se toh bnta hae
Haa, wo typing mistake hua tha. Ab correct kar diye.
Ok sir
In question no. 6 .. A reaction should be Zn + 2NaOH – Na2ZnO2 + H2 but in that reaction 3NaOH. So, please correct it to 2NaOH. Thanks..
Rectified. Thanks for telling me.
Sir acids bases and salts is chapter ka notes milanga kya sir
Haa jald hi upload kar denge.
Sir,
My question is, can I study from oswaal 11th question banks to practice for state board?
Very helpful sir. Thankyou very much. 🙏
You are most welcome 😊
Q-25 ke answer me gypsum ki jagah pe plaster of Paris likha hona chahiye reaction me, sir plz correct it
Naa, Gypsum hi hoga…
Us this questions enough for revision for chapters as well as for the boards?
Us this questions enough for revision for chapters as well as for the boards?
I want to know…
🫡🫡🫡🫡
thank you for this sir lots of love from telangana